Gallagher v. Abbott Laboratories
United States Court of Appeals for the Seventh Circuit
269 F.3d 806 (2001)
- Written by John Caddell, JD
Facts
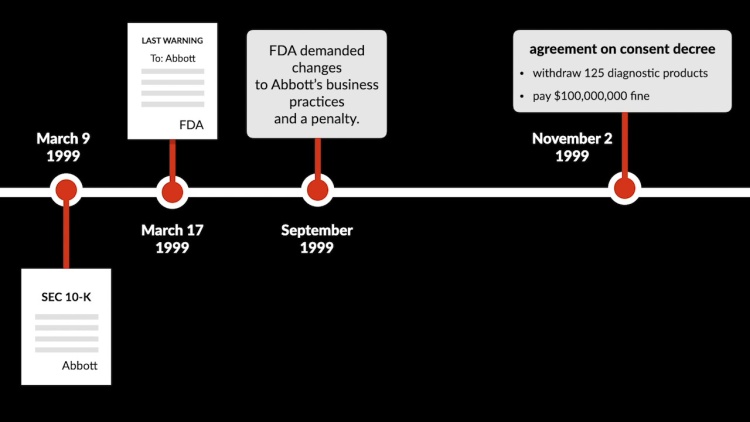
The Food and Drug Administration (FDA) repeatedly issued warnings to the diagnostic division of Abbott Laboratories (Abbott Labs) (defendant). Abbott Labs had filed an annual Securities and Exchange Commission (SEC) 10-K report on March 9, 1999, detailing the corporation’s financial status. On March 17, 1999, the FDA sent Abbott Labs a letter that threatened severe consequences if Abbott Labs did not comply with regulatory requirements. Subsequently, on September 29, 1999, Abbott Labs issued a public statement explaining the FDA’s arguments and indicating that Abbott Labs and the FDA were engaging in settlement discussions. Abbott Labs and the FDA reached an agreement on November 2, 1999, that required Abbott Labs to remove numerous products from the market and pay a large civil fine. Lena Gallagher (plaintiff), representing a class of investors, sued Abbott Labs, arguing that Abbott Labs had committed fraud by deferring the public revelation of information. The district court dismissed the complaint, and Gallagher appealed.
Rule of Law
Issue
Holding and Reasoning (Easterbrook, J.)
What to do next…
Here's why 907,000 law students have relied on our case briefs:
- Written by law professors and practitioners, not other law students. 47,100 briefs, keyed to 996 casebooks. Top-notch customer support.
- The right amount of information, includes the facts, issues, rule of law, holding and reasoning, and any concurrences and dissents.
- Access in your classes, works on your mobile and tablet. Massive library of related video lessons and high quality multiple-choice questions.
- Easy to use, uniform format for every case brief. Written in plain English, not in legalese. Our briefs summarize and simplify; they don’t just repeat the court’s language.